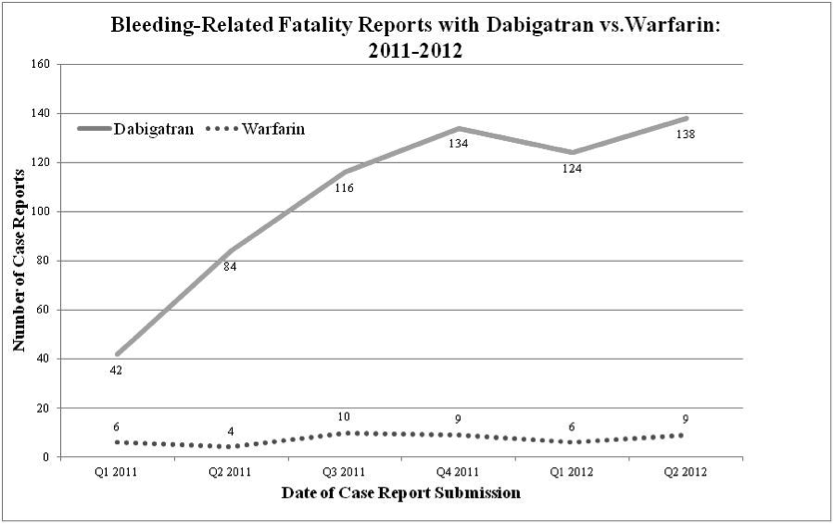
A FDA report presented at the 2013 American College of Cardiology (ACC) 2013 Scientific Sessions has suggested a much higher case fatality rate than that reported in other major clinical trials of the drug. For the study, reports of bleeding with dabigatran or warfarin submitted to the FDA between January 1, 2010, and June 30, 2012 were examined:
Adverse drug reactions relating to warfarin or dabigatran reported to FDA
| End point | Warfarin | Dabigatran |
| Total adverse events reported (n) | 4524 | 12 581 |
| Bleeding events, n (%) | 590 (13) | 2453 (19) |
| % female | 54 | 48 |
| Age (y) | 68.5 | 75 |
| Weight (kg) | 85 | 82 |
Of the 2453 dabigatran bleeding adverse events reported to the FDA, 393 (16%) were fatal, almost double the case fatality rate of patients who bled in the five phase 3 trials of the drug, he noted.
Bleeding events reported to the FDA
| Bleeding event | Warfarin | Dabigatran |
| Total bleeds (n) | 590 | 2453 |
| GI bleeds, n (%) | 162 (27%) | 1352 (55%) |
| Intracranial bleed, n (%) | 69 (12%) | 280 (11%) |
| Bleed fatalities, n (%) | 47 (8%) | 393 (16%) |
Thirty-day mortality after the first major bleed in five phase 3 trials was 13% for warfarin-treated patients and 9% for dabigatran-treated patients.
Reference:
McConeghy K, Bress A, Wing C. Reports of bleeding-related fatalities with dabigatran and warfarin: An analysis using the Food and Drug Administration adverse events reporting system. American College of Cardiology 2013 Scientific Sessions; March 10, 2013; San Francisco, CA.

Comments 1
Pingback: Chronic anticoagulation in atrial fibrillation - Cardiac Health