Subatmospheric pressure has multiple beneficial effects on wound healing in animal models. However, clinical evidence of its superiority over conventional wound dressing techniques for all wound types has not been proven. The available randomized trials have significant heterogeneity in the nature of wounds treated and in primary and secondary end points, making rigorous comparisons difficult and limiting the ability to generalize their results.
The general mechanism of action of NPWT, its clinical uses and contraindications, placement and management of the device, and efficacy in specific clinical applications will be reviewed here.
DEVICE AND PLACEMENT — Commercially-available systems for negative pressure wound therapy (NPWT) include the vacuum-assisted closure (V.A.C.™ therapy) device (KCI, San Antonio, Texas) and the Chariker-Jeter™ wound sealing kit (Smith and Nephew PLC, London, UK). V.A.C.™ therapy is the most widely-studied system in randomized trials.
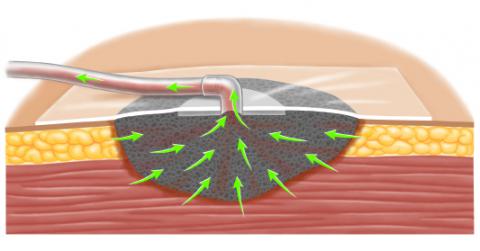
NPWT systems consist of an open-pore polyurethane ether foam sponge, semiocclusive adhesive cover, fluid collection system, and suction pump [2]. The following steps are involved in placing the device:
- The foam sponge is trimmed to fit the size of the open wound and placed into the wound, taking care that the foam does not extend beyond the margin of the wound.
- The foam is then secured beneath an adhesive sheet. A hole is cut in the adhesive and a suction port (more than one may be used) with tubing placed; the tubing extends to a disposable collection canister.
- A portable pump is connected to the suction tubing. The pump applies -50 to -175 mmHg of continuous or intermittent suction, which reduces the volume of the foam by up to 80 percent [3]. The porous nature of the polyurethane foam evenly distributes subatmospheric pressure to the surface of the wound, and provides a conduit for fluid removal from the wound surface to the collection system.
When fragile structures are present within the wound, they should be protected with an interposition layer placed beneath the foam. The interposition layer should slide easily over the tissue. Examples of materials used as interposition layers include mesh (eg, Vicryl®) or petrolatum gauze [2].
Dressing changes — The dressing and tubing are typically changed every 24 to 36 hours depending upon the clinical situation. The device is turned off and the semi-occlusive dressing is removed. The sponge is carefully removed. If it is adherent to the underlying granulation tissue, the sponge can be soaked with saline and allowed to sit for a few minutes before removal. If pain is excessive during sponge removal, the sponge can be soaked with topical Xylocaine without epinephrine, prior to its removal either directly or via the suction tubing (suction off) [2]. Pain is controlled more effectively when administered prior to the dressing change.
Wounds treated with NPWT may become malodorous, and infection may be suspected; however, bacterial counts typically remain low [2]. Hydrotherapy at the time of dressing changes, or the placement of a silver-containing interposition layer (eg, Aquacel® silver) can help reduce odor. Alternatively, NPWT can be withheld for a day or two.
Pain management — Most patients do not complain of significant pain with NPWT between dressing changes. However, appropriate pain medication should be given as the need arises (eg, acetaminophen with codeine).
Patient training and counseling — The patient should receive training on how to use the device, and be comfortable managing the device at home. Patients should be monitored frequently in an appropriate care setting by a trained practitioner. The patient should be informed of the potential complications associated with the device, and of the necessity to seek medical assistance immediately if bleeding is observed. (See ‘Complications’ below.)
MECHANISM OF ACTION — Negative pressure wound therapy (NPWT) accelerates wound healing. Normal wound healing progresses through the following phases: hemostasis, inflammation, proliferation, and remodeling.
Both systemic and local wound factors can contribute to delayed wound healing. Systemic factors (eg, poor nutrition, wound ischemia) should be identified and corrected to the extent that is possible. (See “Treatment of chronic lower extremity critical limb ischemia” and “Overview and management of lower extremity chronic venous disease” and “Overview of perioperative nutritional support”, section on ‘Nutritional assessment in the surgical patient’.)
Local wound factors that interfere with normal healing include desiccation, tissue edema, excessive exudate, poor tissue apposition (eg, grafts and flaps), and wound infection. Stagnant fluid is associated with cytogenetic factors that impede wound healing [4-10].
Animal studies have shown that subatmospheric pressure improves the local wound environment through both direct and indirect effects; these effects accelerate healing and reduce the time to wound closure [11-15].
Direct effects — The semipermeable dressing of the negative pressure system maintains a moist and warm environment that is stable and more conducive to wound healing. The closed system generates a pressure gradient between the wound and suction canister that promotes fluid transport, first from the wound bed, and then from the interstitial space, reducing wound edema.
The open porous structure of the foam transmits the negative pressure to the wound surface. The wound deforms, drawing the edges of the wound together and firmly apposing any skin grafts or flaps that are present [2,16]. The degree of tissue deformation depends upon the stiffness of the tissue; scar tissue has less mobility and will have less of a response. Tissue deformation is an important stimulus for tissue remodeling mediated at the cellular level [16,17].
Indirect effects — Negative pressure wound therapy (NPWT) is associated with a variety of indirect effects that promote wound healing [11]. These include:
- Increased blood flow — Subatmospheric pressure increases blood flow [12,18-20]. However, care must be taken, since excessive negative pressure (more than -175 mmHg) decreases blood flow [18].
- Diminished inflammatory response — Reductions in systemic (eg, interleukins, monocytes) and local mediators of inflammation have been demonstrated in experimental models [21]. In humans, decreased matrix metalloproteinase activity has been documented in patients treated with NPWT [22].
- Altered bacterial burden — A reduction in bacterial burden was noted during the first clinical experiments evaluating NPWT, but the results were variable in later studies [12,23,24]. In a randomized trial, for example, both increasing and decreasing bacterial loads were seen depending upon the initial culture result [24].
- Changes in wound biochemistry — Mechanical deformation alters the local environment through the process of mechanotransduction (conversion of mechanical stimulus into chemical activity). In vitro, stretch increases human fibroblast growth and migration [17]. In an animal wound closure model, NPWT increased collagen organization, and increased the expression of vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2) [25]. The rate of wound closure was significantly higher in the NPWT group compared to gauze controls at day seven (54 versus 43 percent).
INDICATIONS — The application of negative pressure to assist in wound healing was first described in the management of soft tissue injury in association with open fracture [26]. The demonstration of beneficial effects in animal models spurred the development of the negative pressure wound systems that are widely available [12,27].
Negative pressure wound therapy (NPWT, also called vacuum-assisted wound closure) has been applied to a wide range of clinical situations, including the open abdomen, following surgical debridement of acute or chronic wounds (eg, orthopedic, necrotizing infection, pressure ulcer), diabetic foot ulcers, and reconstructive surgery (eg, burns, skin graft, muscle flap) [1,28].
Advantages — Compared to traditional wound care modalities, NPWT offers several clinical advantages compared to usual care.
- Traditional therapy consists of moist saline dressings that are changed up to three times daily. If too much time elapses between dressing changes, the gauze may become painfully adherent and its removal may debride desirable granulation tissue as well as devitalized tissue. Much of the pain associated with wound care occurs during dressing changes. In contrast, NPWT dressings are changed once every two to three days and anticipated pain can be managed preemptively. (See ‘Device and placement’ above and ‘Dressing changes’ above.)
- Compared to other forms of wound dressing, NPWT is easier to tailor and maintain in position. Almost every configuration of wound including circumferential extremity wounds (ie, degloving injuries), and wounds located in proximity to orthopedic fixation frames can be managed with relative ease [29-31]. As a result, NPWT may allow less complex modes of reconstructive surgery. Complex wounds that required a pedicle flap may, after NPWT, be converted to a wound requiring a rotation flap or skin graft [2].
- Accelerated wound healing with NPWT significantly reduces the time to wound closure in diabetic patients, returning these patients to baseline more quickly and improving quality of life [32]. (See ‘Chronic wounds’ below.)
- Reduced complexity of subsequent reconstructive procedures.
Disadvantages — From the patient’s perspective, the main disadvantage of NPWT is the need to carry the portable pump.
NPWT systems are more expensive than traditional wound dressings. However, the overall cost of wound care depends upon the frequency of dressing changes, need for skilled nursing care, and duration of treatment. Significant clinical reductions in time to wound closure would be needed to offset the increased cost of the device and special supplies for NPWT to be cost-effective, but data are limited.
Contraindications — NPWT should not be used when any of the following are present [33]:
- Exposed vital structures — NPWT, in the presence of exposed organs, blood vessels, or vascular grafts, increases the risk for tissue erosion, which can lead to enteric fistula or hemorrhage [14,34]. NPWT is generally avoided until an intervening granulation layer or tissue flap or graft provides coverage. Although some clinicians report success using barrier dressings, caution is advised when implementing this practice. (See ‘Device and placement’ above and‘Complications’ below.)
- Ongoing infection — Active infection should be treated prior to using NPWT. (See “Cellulitis and erysipelas” and “Necrotizing soft tissue infections”.)
- Devitalized tissue — Inadequate debridement with the presence of devitalized soft tissue or bone increases the risk for infection [14] (see ‘Complications’ below).
- Malignant tissue — As with normal tissues, growth of malignant tissue is promoted in the presence of subatmospheric pressure. Malignant tissue is also more friable and prone to bleeding [2,14]. (See ‘Bleeding’ below.)
- Fragile skin — Patients with fragile skin due to age, chronic corticosteroid use, or collagen vascular disorder should not be treated with NPWT. Shearing forces at the wound margin can lead to skin avulsion and necrosis.
- Adhesive allergy — NPWT requires an adequate seal to maintain the applied suction. The adhesive cover typically overlaps the skin 4 to 5 cm with a significant amount of adhesive in contact with the patient’s skin. Sensitive patients can develop shearing of the skin and bullae formation.
- Ischemic wounds — Although not absolutely contraindicated, no benefit has been demonstrated with the use of NPWT in patients with ischemic wounds [11]. The application of negative pressure to these wounds would be expected worsen tissue ischemia. (See ‘Chronic wounds’ below.)
CLINICAL APPLICATIONS
Overall efficacy — Multiple systematic reviews have sought to evaluate the efficacy of negative pressure wound therapy (NPWT), but none have reached definitive conclusions [3,28,35-42]. The most rigorous of these reviews identified eight randomized trials that met three inclusion criteria: randomized, chronic wound, and specific endpoints [35]. NPWT was used to treat diabetic ulcers, pressure (decubitus) ulcers, or chronic lower extremity ulcers. The primary endpoints were one of the following:
- Time to complete healing
- Days to reach 50 percent of initial wound volume
- Percent reduction in wound surface area
- Percent decrease in wound length, width, depth or volume
- Days until ready for surgery
Because only one study was available for each endpoint, comparisons could not be made. Even if a meta-analysis were able to be performed, the question of whether chronic (or acute) wounds of differing mechanisms in different patient populations should be compared to each other.
Another approach evaluates the available data (observational and randomized trials) for a specific clinical situation such as wounds from acute injury (eg, trauma, burns, surgical debridement) [28,38,39], wounds in diabetic patients (eg, ulcers, postoperative wounds) [36,41], open abdomen [43], and open sternum (eg, following debridement) [40]. Available trials are presented for these individual clinical situations.
Acute wounds — Acute wounds are often traumatic, but can also be due to surgical debridement of infected or necrotic tissue. Management of necrotizing soft tissue infection requires extensive and repeated surgical debridement. The debrided regions often present a wound dressing challenge due to anatomic location (eg, Fournier’s gangrene), the size of the tissue defect or the patient’s body habitus. (See “Necrotizing soft tissue infections”, section on ‘Surgery’.)
The open wound that results is often substantial. For most patients, the question is generally when, not if, their wounds will heal. The time interval required until either secondary closure can be performed or healing by secondary intention occurs is variable and depends upon the size of the defect, and the patient’s overall clinical status (eg, other injuries, nutrition, comorbidities).
NPWT dressings can be applied immediately following operative debridement, which simplifies postoperative wound care. The ability of the foam and adhesive dressing to conform to almost any wound contour, shape, or size contributes to the success of NPWT, as detailed in case reports, in these complex wounds [43-46]. NPWT can also be used in conjunction with skin grafts or flaps, which are frequently needed to cover tissue defects. (See ‘Skin graft/flap fixation’ below.)
For acute open wounds, NPWT is associated with a reduced time to wound closure [23,24]. As an example, one trial randomly assigned 54 patients with open wounds to receive either NPWT or moist saline dressings [24]. The NPWT group had healthier-appearing wounds and significantly faster reduction of the wound surface area (3.8 versus 1.7 percent per day).
NPWT has also been used to manage acute wounds resulting from lower extremity fasciotomy, degloving injury, open amputation, and complex traumatic wounds with exposed tendon, bone, or orthopedic hardware. These wounds are typically large and difficult to dress.
In trauma patients, a systematic review of the literature did not identify any randomized trials; however, the available observational studies suggest that NPWT is safe and with an efficacy comparable to standard dressings [39]. The primary clinical advantage of NPWT in the trauma population is its ease of application, decreased number of dressing changes, and reduction in the complexity of subsequent reconstructive procedures [27,47-50].
NPWT may have a particular role in the treatment of burn wounds. Impairment of blood flow in the zone of stasis may lead to burn wound progression (ie, partial thickness burn becomes full thickness burn). In animal models, subatmospheric pressure increases burn wound perfusion and limits this progression [51]. (See ‘Mechanism of action’ above.)
Anecdotal case reports and small case series have reported NPWT in the treatment of acute burn wounds [38,52,53]. Two studies have looked at bilateral hand burns as a model: one hand is treated with conventional dressings and the other with NPWT [52,53]. A significant clinical advantage of the NPWT group was the ability to position the hand without the need for additional splinting. These preliminary studies have demonstrated the safety and feasibility of NPWT in burn patients.
Chronic wounds — NPWT may improve the healing of some types of chronic wounds/ulceration provided that they are well vascularized [30,39,54]. Patients with extremity wounds and inadequate peripheral pulses should undergo noninvasive vascular testing to confirm adequate perfusion prior to instituting NPWT, especially patients with diabetes or other risk factors for peripheral artery disease. (See “Noninvasive diagnosis of arterial disease”.)
Venous stasis ulcers are uncommonly confused with other types of chronic ulcers. While these ulcers are associated with significant wound edema and exudate, they are managed with local wound care and compression therapy. NPWT is not a part of routine management. (See “Medical management of lower extremity chronic venous disease”.)
Compared with conventional dressing changes, NPWT reduces time to closure of diabetic foot ulcers and wounds resulting from diabetic foot surgery. In this population of patients, NPWT is also associated with shorter length of hospitalization, decreased complication rates, and reduced costs. The use of NPWT in the management of diabetic foot lesions is discussed in detail separately. (See “Management of diabetic foot lesions”.)
Three randomized trials have evaluated the use of NPWT as an adjunctive therapy for the management of pressure (decubitus) ulcers [30,31,55]. No statistically significant differences were identified with respect to quantitative wound healing measures (eg, wound surface area reduction). However, NPWT improved patient comfort and was less labor intensive [56]. (See “Treatment of pressure ulcers”.)
Skin graft/flap fixation — NPWT has been used instead of traditional bolstering methods to provide skin graft fixation [28,57]. The NPWT dressing distributes negative pressure uniformly over the surface of the fresh graft, immobilizing the graft with less chance of shearing [58]. Improved qualitative skin graft take and quantitative improvements in skin graft success (eg, reduced number of repeat grafts) have been described in observational studies [47,49,59,60] and two randomized trials [61,62].
In one of the trials, 60 patients were randomly assigned to conventional bolster dressing or NPWT following split thickness skin graft [61]. NPWT was associated with significant reduction in the loss of graft area (zero versus 4.5 cm(2) in the control group) and the median duration of hospitalization (13.5 versus 17 days).
Open abdomen — The abdominal wall of patients undergoing exploration for severe abdominal trauma is frequently left open to facilitate second look operations [43,63]. Reopening the abdomen may also be needed in patients for whom there is a concern for abdominal compartment syndrome. In this patient population, NPWT improves the success of both early and late (>9 days) fascial closure. A general discussion of the management of the open abdomen is found elsewhere. (See“Abdominal compartment syndrome”.)
Open sternum — Post-sternotomy mediastinitis is an uncommon but devastating complication of cardiac surgery with high morbidity and with mortality [28]. Management consists of aggressive debridement, antibiotics and wound care which may include the use of negative pressure wound therapy to manage the open wound while awaiting sternal closure. The use of negative pressure wound therapy in this population is discussed in detail elsewhere. (See “Surgical management of sternal wound complications”, section on ‘Negative pressure wound therapy’.)
COMPLICATIONS — NPWT is generally safe and well tolerated. Complications are most likely to occur when NPWT is applied to patients whose wounds have devitalized tissue or exposed vital structures (eg, organs, blood vessels, vascular grafts). (See ‘Contraindications’ above.)
Bleeding — Bleeding is the most serious complication of NPWT and can occur in hospitals, long-term care facilities, and at home [34]. Minor bleeding during dressing changes due to granulation tissue at the base of the wound is common and is best managed with firm pressure to the wound surface. Severe hemorrhage can occur during removal of foam that has become adherent to the granulation tissue below, especially in patients who are anticoagulated, or in patients with exposed vessels or vascular grafts. In patients with severe bleeding, direct pressure should be applied and emergency services contacted. Surgery may be needed to control bleeding.
Infection — Infection related to the use of NPWT is often due to prior wound infection that was inadequately controlled prior to initiating NPWT. When infection is suspected (eg, fever, erythema, cellulitis), the NPWT dressing in discontinued, the wound irrigated and debrided, wound cultures obtained, and empiric antibiotics initiated. (See “Cellulitis and erysipelas”, section on ‘Treatment’.)
Enterocutaneous fistula — Scattered case reports suggest that NPWT may expedite control and closure of postoperative enterocutaneous fistula. However, NPWT is also more likely to cause enteric fistula formation [64-68].
SUMMARY AND RECOMMENDATIONS
- Negative pressure wound therapy (NPWT), also called vacuum-assisted closure, is an adjunctive therapy used in the management of open wounds that applies subatmospheric pressure to the wound surface. The wound care system consists of an open-cell foam dressing, semiocclusive adhesive cover, fluid collection system and suction pump. (See ‘Introduction’ above and ‘Device and placement’ above.)
- NPWT exerts its effect through direct and indirect effects of subatmospheric pressure. These effects include stabilization of the wound environment, increased blood flow and deformation of the wound. Deformation is a powerful stimulus for cellular processes that stimulate granulation tissue and accelerate wound healing. (See ‘Mechanism of action’ above.)
- NPWT has several advantages over traditional wound management, including simplification of wound care (primarily through a reduced number of dressing changes and ease of tailoring the dressing), accelerated wound healing, and reduction in the complexity of subsequent reconstructive procedures. (See ‘Clinical applications’ above.)
- NPWT has been utilized in the treatment of wounds from acute injury (eg, trauma, burns, surgical debridement), wounds in diabetic patients (eg, ulcers, postoperative wounds), open abdomen [43], and open sternum (eg, following debridement). High quality data supporting the use of NPWT are available only for the management of diabetic foot wounds. (See ‘Clinical applications’ above and “Management of diabetic foot lesions”.)
- We recommend not using NPWT if perfusion to the wound is inadequate (Grade 1C). NPWT can cause or worsen tissue ischemia. Other contraindications to negative pressure wound therapy include the presence of vital structures within the wound, including organs, blood vessels, or vascular grafts; ongoing infection (ie, cellulitis, osteomyelitis); devitalized tissue in the wound; malignancy in the wound; skin or tissue fragility; and adhesive allergy (see ‘Contraindications’above).Capobianco CM, Zgonis T. An overview of negative pressure wound therapy for the lower extremity. Clin Podiatr Med Surg 2009; 26:619.
- Venturi ML, Attinger CE, Mesbahi AN, et al. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) Device: a review. Am J Clin Dermatol 2005; 6:185.
- Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H. A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg 2008; 95:685.
- Bucalo B, Eaglstein WH, Falanga V. Inhibition of cell proliferation by chronic wound fluid. Wound Repair Regen 1993; 1:181.
- Banwell PE. Topical negative pressure therapy in wound care. J Wound Care 1999; 8:79.
- Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol 1993; 101:64.
- Sapico FL, Ginunas VJ, Thornhill-Joynes M, et al. Quantitative microbiology of pressure sores in different stages of healing. Diagn Microbiol Infect Dis 1986; 5:31.
- Hunt TK. The physiology of wound healing. Ann Emerg Med 1988; 17:1265.
- Falanga V. Growth factors and chronic wounds: the need to understand the microenvironment. J Dermatol 1992; 19:667.
- Genecov DG, Schneider AM, Morykwas MJ, et al. A controlled subatmospheric pressure dressing increases the rate of skin graft donor site reepithelialization. Ann Plast Surg 1998; 40:219.
- Orgill DP, Manders EK, Sumpio BE, et al. The mechanisms of action of vacuum assisted closure: more to learn. Surgery 2009; 146:40.
- Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997; 38:553.
- Pollak AN. Use of negative pressure wound therapy with reticulated open cell foam for lower extremity trauma. J Orthop Trauma 2008; 22:S142.
- Whelan C, Stewart J, Schwartz BF. Mechanics of wound healing and importance of Vacuum Assisted Closure in urology. J Urol 2005; 173:1463.
- Scherer SS, Pietramaggiori G, Mathews JC, et al. The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg 2008; 122:786.
- Urschel JD, Scott PG, Williams HT. The effect of mechanical stress on soft and hard tissue repair; a review. Br J Plast Surg 1988; 41:182.
- Nishimura K, Blume P, Ohgi S, Sumpio BE. Effect of different frequencies of tensile strain on human dermal fibroblast proliferation and survival. Wound Repair Regen 2007; 15:646.
- Kairinos N, Voogd AM, Botha PH, et al. Negative-pressure wound therapy II: negative-pressure wound therapy and increased perfusion. Just an illusion? Plast Reconstr Surg 2009; 123:601.
- Ichioka S, Watanabe H, Sekiya N, et al. A technique to visualize wound bed microcirculation and the acute effect of negative pressure. Wound Repair Regen 2008; 16:460.
- Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 2001; 47:547.
- Norbury, K. Vacuum-assisted closure therapy attenuates the inflammatory response in porcine acute wound healing model. Wounds 2007; 19:97. Available online at: www.woundsresearch.com/article/7180 (Accessed on June 15, 2010).
- Greene AK, Puder M, Roy R, et al. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 2006; 56:418.
- Mouës CM, van den Bemd GJ, Heule F, Hovius SE. Comparing conventional gauze therapy to vacuum-assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg 2007; 60:672.
- Mouës CM, Vos MC, van den Bemd GJ, et al. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004; 12:11.
- Jacobs S, Simhaee DA, Marsano A, et al. Efficacy and mechanisms of vacuum-assisted closure (VAC) therapy in promoting wound healing: a rodent model. J Plast Reconstr Aesthet Surg 2009; 62:1331.
- Fleischmann W, Strecker W, Bombelli M, Kinzl L. [Vacuum sealing as treatment of soft tissue damage in open fractures]. Unfallchirurg 1993; 96:488.
- Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997; 38:563.
- Bovill E, Banwell PE, Teot L, et al. Topical negative pressure wound therapy: a review of its role and guidelines for its use in the management of acute wounds. Int Wound J 2008; 5:511.
- Braakenburg A, Obdeijn MC, Feitz R, et al. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 2006; 118:390.
- Joseph, E, Hamori, CA, Bergman, S, et al. A prospective randomized trial of vacuum-assisted closure versus standard therapy of chronic non-healing wounds. Wounds 2000; 12:60. Available online at: www.medscape.com/viewarticle/407550 (Accessed on June 15, 2010).
- Ford CN, Reinhard ER, Yeh D, et al. Interim analysis of a prospective, randomized trial of vacuum-assisted closure versus the healthpoint system in the management of pressure ulcers. Ann Plast Surg 2002; 49:55.
- Blume PA, Walters J, Payne W, et al. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008; 31:631.
- Sumpio BE, Allie DE, Horvath KA, et al. Role of negative pressure wound therapy in treating peripheral vascular graft infections. Vascular 2008; 16:194.
- White RA, Miki RA, Kazmier P, Anglen JO. Vacuum-assisted closure complicated by erosion and hemorrhage of the anterior tibial artery. J Orthop Trauma 2005; 19:56.
- Ubbink DT, Westerbos SJ, Evans D, et al. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev 2008; :CD001898.
- Noble-Bell G, Forbes A. A systematic review of the effectiveness of negative pressure wound therapy in the management of diabetes foot ulcers. Int Wound J 2008; 5:233.
- Expert Working Group. Vacuum assisted closure: recommendations for use. A consensus document. Int Wound J 2008; 5 Suppl 4:iii.
- Wasiak J, Cleland H. Topical negative pressure (TNP) for partial thickness burns. Cochrane Database Syst Rev 2007; :CD006215.
- Kanakaris NK, Thanasas C, Keramaris N, et al. The efficacy of negative pressure wound therapy in the management of lower extremity trauma: review of clinical evidence. Injury 2007; 38 Suppl 5:S9.
- Sjögren J, Malmsjö M, Gustafsson R, Ingemansson R. Poststernotomy mediastinitis: a review of conventional surgical treatments, vacuum-assisted closure therapy and presentation of the Lund University Hospital mediastinitis algorithm. Eur J Cardiothorac Surg 2006; 30:898.
- Pham CT, Middleton PF, Maddern GJ. The safety and efficacy of topical negative pressure in non-healing wounds: a systematic review. J Wound Care 2006; 15:240.
- Mendonca DA, Papini R, Price PE. Negative-pressure wound therapy: a snapshot of the evidence. Int Wound J 2006; 3:261.
- Stevens P. Vacuum-assisted closure of laparostomy wounds: a critical review of the literature. Int Wound J 2009; 6:259.
- Kirby JP, Fantus RJ, Ward S, et al. Novel uses of a negative-pressure wound care system. J Trauma 2002; 53:117.
- Garner GB, Ware DN, Cocanour CS, et al. Vacuum-assisted wound closure provides early fascial reapproximation in trauma patients with open abdomens. Am J Surg 2001; 182:630.
- Hofmann P, Friess P, Findeisen M, Tomcik P. [Case report of successful therapy of necrotizing fasciitis using a device of vacuum assisted closure]. Zentralbl Chir 2006; 131 Suppl 1:S72.
- Barendse-Hofmann MG, van Doorn L, Steenvoorde P. Circumferential application of VAC on a large degloving injury on the lower extremity. J Wound Care 2009; 18:79.
- DeFranzo AJ, Argenta LC, Marks MW, et al. The use of vacuum-assisted closure therapy for the treatment of lower-extremity wounds with exposed bone. Plast Reconstr Surg 2001; 108:1184.
- DeFranzo AJ, Marks MW, Argenta LC, Genecov DG. Vacuum-assisted closure for the treatment of degloving injuries. Plast Reconstr Surg 1999; 104:2145.
- Meara JG, Guo L, Smith JD, et al. Vacuum-assisted closure in the treatment of degloving injuries. Ann Plast Surg 1999; 42:589.
- Morykwas MJ, David LR, Schneider AM, et al. Use of subatmospheric pressure to prevent progression of partial-thickness burns in a swine model. J Burn Care Rehabil 1999; 20:15.
- Molnar, JA. Application of negative pressure wound therapy to thermal injury. Ostomy wound management 2004; 50 (4A Suppl):17S.
- Kamolz LP, Andel H, Haslik W, et al. Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns 2004; 30:253.
- Vuerstaek JD, Vainas T, Wuite J, et al. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006; 44:1029.
- Wanner MB, Schwarzl F, Strub B, et al. Vacuum-assisted wound closure for cheaper and more comfortable healing of pressure sores: a prospective study. Scand J Plast Reconstr Surg Hand Surg 2003; 37:28.
- Mandal A. Role of topical negative pressure in pressure ulcer management. J Wound Care 2007; 16:33.
- Jeschke MG, Rose C, Angele P, et al. Development of new reconstructive techniques: use of Integra in combination with fibrin glue and negative-pressure therapy for reconstruction of acute and chronic wounds. Plast Reconstr Surg 2004; 113:525.
- Schneider AM, Morykwas MJ, Argenta LC. A new and reliable method of securing skin grafts to the difficult recipient bed. Plast Reconstr Surg 1998; 102:1195.
- Molnar JA, DeFranzo AJ, Marks MW. Single-stage approach to skin grafting the exposed skull. Plast Reconstr Surg 2000; 105:174.
- Scherer LA, Shiver S, Chang M, et al. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg 2002; 137:930.
- Llanos S, Danilla S, Barraza C, et al. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg 2006; 244:700.
- Moisidis E, Heath T, Boorer C, et al. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 2004; 114:917.
- Rotondo MF, Schwab CW, McGonigal MD, et al. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma 1993; 35:375.
- Schecter WP, Hirshberg A, Chang DS, et al. Enteric fistulas: principles of management. J Am Coll Surg 2009; 209:484.
- Cro C, George KJ, Donnelly J, et al. Vacuum assisted closure system in the management of enterocutaneous fistulae. Postgrad Med J 2002; 78:364.
- Alvarez AA, Maxwell GL, Rodriguez GC. Vacuum-assisted closure for cutaneous gastrointestinal fistula management. Gynecol Oncol 2001; 80:413.
- Rao M, Burke D, Finan PJ, Sagar PM. The use of vacuum-assisted closure of abdominal wounds: a word of caution. Colorectal Dis 2007; 9:266.
- Fischer JE. A cautionary note: the use of vacuum-assisted closure systems in the treatment of gastrointestinal cutaneous fistula may be associated with higher mortality from subsequent fistula development. Am J Surg 2008; 196:1.