Introduction
Aortic dissection results from a tear in the inner layer of the aortic wall (called intimal tear). Blood pumped from the heart into the aorta pushes the layers further apart in an upstream or downstream direction (or both, see below) and block side branches that may include
- Your coronary arteries, causing a heart attack
- Arteries to your brain , causing a stroke
- Spinal cord, causing paralysis
- Arteries in your abdomen, causing damage to organs like your kidneys, intestine
- Arteries to your arms & legs resulting in cold, painful extremities because of the lack of blood.
Aortic dissection can also rupture into the pericardium (heart sack). Blood that enters will press on the heart, causing cardiac tamponade; dissection into the aortic root leading to severe aortic valve leakage.
The majority of dissections (95%) occur in two places. The most common site is within several centimeters of the aortic valve, and the second most common is just beyond the artery to your left arm.
Causes
Conditions commonly associated with aortic dissection include the following:
● Hypertension
An abrupt, transient, severe increase in blood pressure has been associated with acute aortic dissection through various mechanisms. Crack cocaine, which may cause momentary hypertension, accounted for 37 percent of dissections in a published report.
High-intensity weight lifting or other strenuous resistance training can also cause a transient elevation in blood pressure and has been reported as an antecedent.
● Genetically mediated connective tissue disorders (eg, Marfan syndrome, Ehlers-Danlos syndrome)
● Preexisting aortic aneurysm
● Bicuspid aortic valve
● Aortic coarctation
● Inflammatory diseases – Inflammatory diseases that cause vasculitis (giant cell arteritis, Takayasu arteritis, rheumatoid arthritis, syphilitic aortitis) are associated with thoracic aortic aneurysm/dissection .
● Trauma – Trauma rarely causes a classic dissection but can induce a localized tear in the region of the aortic isthmus. More commonly, chest trauma from acute deceleration (as in a motor vehicle accident) results in aortic rupture or transection :
Signs and Symptoms
In an analysis of patients with acute chest and/or back pain almost 100 % of acute aortic dissections could be diagnosed based upon three clinical features :
● Abrupt onset of thoracic or abdominal pain with a sharp, tearing, and/or ripping character
● A variation in pulse (absence of a proximal extremity or carotid pulse) and/or blood pressure (>20 mmHg difference between the right and left arm)
● Chest X-ray changes with widening of the aorta
Diagnosis
TransEsophageal Echocardiography (TEE) in Aortic dissection (from medicaldump.com)
Aortic dissection on noninvasive imaging

Representative transesophageal echocardiograms (TEE) (panels A and B) and computed tomograms (CT) (panels C and D) of a section of the descending aorta in distal aortic dissection. Panels A and C are acute studies, while B and D are from follow-up evaluation. Both TEE and CT scan show persistence of the intimal flap forming the true lumen (TL) and a markedly dilated false lumen (FL) with the development of thrombi.
Aortogram showing aortic rupture with pseudoaneurysm

The aortogram, obtained following administration of contrast material into the aortic root, demonstrates a focal pseudoaneurysm (arrow) arising from the proximal descending aorta immediately distal to the origin of the left subclavian artery (small arrow). This traumatic tear of the aorta occurred during a motor vehicle accident.
Treatment
Treatment for dissection depends on its location, symptoms and findings.
If the thoracic aortic aneurysm is small and not causing any symptoms, your physician may recommend “watchful waiting.” By closely monitoring your condition with CT or MRI scans every 6-12 months, the aneurysm will be watched for signs of changes.
If you have high blood pressure, your physician will prescribe blood pressure medication to lower your overall blood pressure and the pressure on the weakened area of the aneurysm. Additionally your physician may prescribe a “statin” (or cholesterol lowering medication) to maintain the health of your blood vessels.
Medical Treatment
For patients diagnosed with dissection, acute management includes anti-impulse therapy (see below), pain control and identification of complications that indicate the need for repair.
Subsequent medical management consists of ongoing pain control, medications to maintain heart rate and blood pressure, and lifelong imaging surveillance.
For patients who develop a complicated course, intervention is required despite intensive medical therapy, 20 to 40 percent of patients may progress to require surgical or endovascular intervention.
Supportive care and monitoring — Blood pressure management typically requires intensive care monitoring with an arterial line for strict heart rate and blood pressure control and may also include monitoring central venous pressure and cardiac indices, depending upon the patient’s clinical and hemodynamic status. Routine laboratories are obtained and trended and include complete blood count, electrolytes, coagulation parameters, and D-dimer, as well as markers of malperfusion, including liver function tests (alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase [ALP], bilirubin), renal function tests (blood urea nitrogen, creatinine), and lactate.
For patients with uncomplicated disease, medical therapies are continued until pain abates and heart rate and blood pressure are well controlled. Any increase in pain or recurrence of pain should raise suspicion that the dissection has worsened, prompting repeat cardiovascular imaging. Other clinical features that may indicate the development of malperfusion syndromes include abdominal pain, worsening renal function, peripheral ischemia (leg pain, pallor, diminished/absent pulses) and spinal cord ischemia causing paralysis.
Anti-impulse therapy — Anti-impulse therapy consists of heart rate (<60 beats per minute) and blood pressure (<120/80) control to decrease aortic wall shear stress.
Decreasing the force of myocardial contraction has been shown to slow aortic expansion and possibly rupture after aortic dissection, and this has been the basis for prescribing beta-blockers as first-line antihypertensive therapy for patients with thoracic aortic dissection.
Exercise limitation — Though unproven, avoidance of strenuous physical activity is also recommended for all patients who have had aortic dissection as another method to minimize aortic shear stress.
Identifying associated genetic conditions — Similar with thoracic aortic aneurysm, patients with thoracic aortic dissection, particularly younger patients or those with clinical features or a family history of connective tissue disorders, and others without an identifiable etiology for aortic dissection (eg, severe hypertension, pregnancy/delivery, aortic trauma) should be evaluated for possible underlying genetic or familial disorders such as Marfan syndrome. These may increase the patient’s individual risk of progression or complication].
Surveillance imaging — For patients with aortic dissection with successful medical stabilization, baseline thoracic magnetic resonance (MR) or computed tomographic (CT) angiography prior to discharge, and follow-up examinations at 3, 6, and 12 months and annually thereafter is indicated, even if the patient remains asymptomatic
The following abnormalities can be detected on serial imaging:
●Extension or recurrence of the dissection
●Aneurysmal degeneration
●Malperfusion
MR angiography may be more acceptable for serial studies because it is noninvasive and does not expose patients to iodinated contrast and ionizing radiation as with CT angiography, which are important factors for younger patients who will likely have many years of serial monitoring. Although, due to concerns about long-term retained gadolinium, noncontrast MR angiography is an alternative that should be considered. Alternating CT and MR angiography is a reasonable option for patients with good renal function.
Surgical Repair
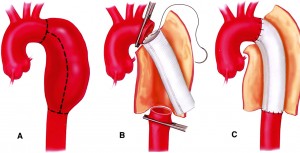
Aortic aneurysm surgically removed and replaced with a graft
If the thoracic aortic aneurysm is large or causing symptoms, you will need prompt treatment to prevent a rupture from occurring. The weakened section of the vessel can be surgically removed and replaced with a graft of artificial material. If the aneurysm is close to the aortic valve (the valve that regulates blood flow from the heart into the aorta), a valve replacement may also be recommended during the procedure.
Repairing the aneurysm surgically is complex and requires an experienced thoracic surgical team. However, neglecting the aneurysm presents a higher risk. Repairing a thoracic aneurysm may require open-chest surgery, general anesthesia and a minimum hospital stay of five days.
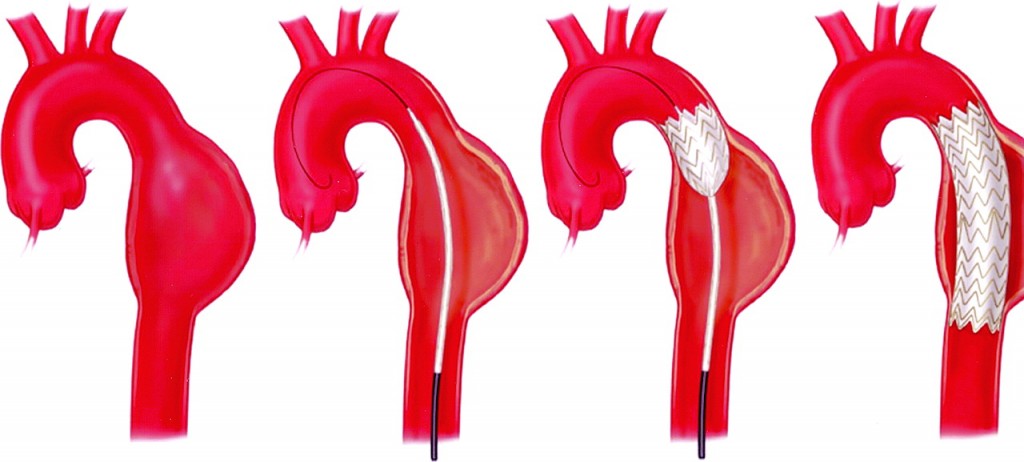
Endovascular Repair
Instead of an open aneurysm repair, your surgeon may consider a newer procedure called an endovascular aneurysm repair (EVAR, TEVAR, TA-EVAR). Endovascular means that surgery is performed inside your aorta using thin, long tubes called catheters. By entering through small incisions in the groin, the catheters are used to guide and deliver a stent-graft through the blood vessels to the site of the aneurysm. The stent graft is then positioned in the diseased segment of aorta to “reline” the aorta like a sleeve to divert blood flow away from the aneurysm.
This endovascular approach is currently used to treat abdominal and descending thoracic aneurysms, and is being evaluated as a treatment for thoracoabdominal and arch aneurysms. While current results are positive, further research is needed to determine who the best candidates for this type of procedure may be.

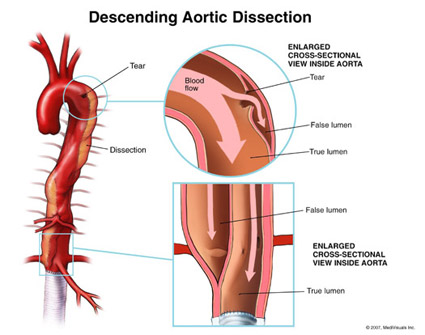
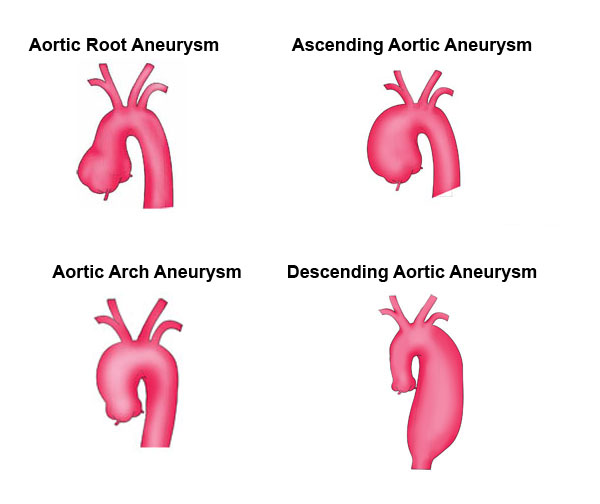
Comments 2
My brother suggested I might like this website He was totally right This post actually made my day You cannt imagine just how much time I had spent for this information Thanks
I had a ruptured asending and decending aortic aneurysm repaired by Denton Cooley in 1981. In 2020 I had a CABG of the left coronary artery and now have developed Stage 3B CKD at 71 years. My cardiologist at the Texas Heart Institute in Houston tells me I need a stent to open the right artery but that has been observed for many years. Should I go for it or just be grateful that I have had this much time and let nature take its course or risk imminent death? My nephrologists, PCP and local (San Antonio) cardiologist think my last TTE (in 3/2022) showed sufficient blood flow and tell me to wait. I had a TEE for the one year follow up for a Watchman about two weeks ago and my THI doctor still wants to proceed. Where can I best have the test results reviewed for an excellent second opinion? I feel pretty much the same as forty years ago, live completely independently, and can travel if necessary – I just tire more easily now as I would expect at my age! My life expectancy probably depends on your response so I thank you for your consideration in advance. My 13.5 year old Border Collie, James, needs me just a little longer too!